Abstract
Introduction: During the clinical development of caplacizumab, safety and tolerability data have been accrued from Phase I, Phase II, and Phase III studies in 3 discrete populations, comprising patients with acquired thrombotic thrombocytopenic purpura (aTTP), patients undergoing percutaneous coronary intervention (PCI; indication is not being pursued), and healthy patients. Given that caplacizumab blocks the interaction of the Willebrand factor (vWF) A1 domain with the GPIb-IX-V platelet receptor, the main expected safety risk is bleeding.
Methods: The objective of this integrated analysis is to characterize the safety and tolerability of caplacizumab based on summaries of data from the pooled Phase I, Phase II, and Phase III studies, with a main focus on the safety data from the Phase II and Phase III studies in the aTTP Population, (i.e., the TITAN and HERCULES studies). Treatment-emergent adverse events (TEAEs) and clinical laboratory evaluations (hematology, biochemistry, and coagulation markers) were evaluated and summarized. Treatment-emergent bleeding was specified as an event of special interest. Data were analyzed during the double-blind (DB)/single-blind (SB) treatment period, and during the overall study period (including the follow-up period) for the aTTP population.
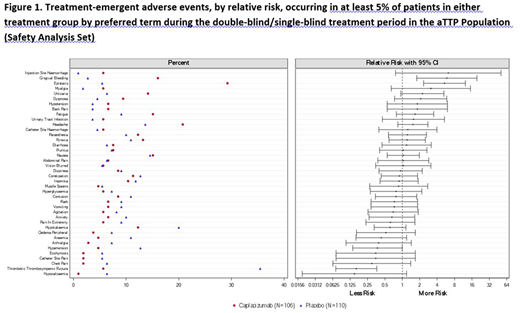
Results: Safety data for caplacizumab have been accrued in 790 patients within the 3 populations (220 aTTP patients, 410 PCI patients, and 160 healthy patients). In the pooled aTTP population, 216 (98.2%) patients received at least one dose of study drug (i.e., Safety Population), 106 patients in the caplacizumab group and 110 patients in the placebo group. The median duration of exposure to study drug was 35.0 days in the caplacizumab group and 32.5 days in the placebo group. Similar percentages of patients were reported with TEAEs in the caplacizumab (96.2%) and placebo (95.5%) groups. Events that occurred more frequently (≥5% difference) in the caplacizumab group vs. placebo were epistaxis (29.2% vs. 5.5%), headache (20.8% vs 13.6%) and gingival bleeding (16.0% vs 2.7%). The majority of these events were mild in intensity. Events that occurred more frequently in the placebo group were TTP (35.5% vs 5.7%), hypokalaemia (20.0% vs 12.3%), and hypertension (12.7% vs 4.7%). Relative risks were calculated for the most frequent TEAEs (Figure 1). Patients in the caplacizumab group had a significantly higher risk of experiencing gingival bleeding and epistaxis. Patients in the placebo group had a significantly higher risk of TTP recurrence and hypertension. Study drug discontinuation due to TEAEs occurred with similar frequencies in the caplacizumab (6.6%) and placebo (10.0%) groups, and were mostly individual events with the exception of TTP and myocardial infarction. A lower percentage of patients experienced SAEs in the caplacizumab group (29.2%) vs. placebo (49.1%). The most frequently reported SAE was TTP in both the caplacizumab (5.7%) and placebo (34.5%) groups. All other SAEs were reported in less than 5% of the patients. A higher percentage of patients experienced bleeding TEAEs in the caplacizumab group (60.4%) than in the placebo group (42.7%). Bleeding TEAEs were mainly mucocutaneous, most were self-limited and the majority resolved. Results from laboratory tests showed a general normalization of key hematology and chemistry parameters over time. Both treatment groups were similar with respect to clinically notable laboratory values, with very few abnormalities reported as TEAEs. The safety profile of caplacizumab observed in other populations was similar to that observed in the aTTP population, with no new safety signals identified. Bleeding TEAEs were the most commonly reported events in healthy patients and PCI patients.
Conclusions: Bleeding TEAEs, chiefly epistaxis and gingival bleeding, were the most common TEAEs in patients treated with caplacizumab. Results from laboratory tests confirmed the safety profile of caplacizumab. No new safety signals were identified in the other populations studied. This integrated analysis has shown that caplacizumab is well tolerated and has a favorable safety profile.
Knöbl:Ablynx: Consultancy, Other: Member of Advisory Board. Scully:Novartis: Honoraria, Other: Member of Advisory Board, Speakers Bureau. Cataland:Alexion: Research Funding; Shire: Consultancy; Ablynx: Consultancy, Other: Member of Advisory Board. Peyvandi:Shire: Speakers Bureau; Octapharma US: Honoraria; Sobi: Speakers Bureau; Grifols: Speakers Bureau; Roche: Speakers Bureau; Kedrion: Consultancy; Ablynx: Other: Member of Advisory Board, Speakers Bureau; Grifols: Speakers Bureau; Sobi: Speakers Bureau; Novo Nordisk: Speakers Bureau; Novo Nordisk: Speakers Bureau; Grifols: Speakers Bureau; Novo Nordisk: Speakers Bureau; Octapharma US: Honoraria; Octapharma US: Honoraria; Kedrion: Consultancy; Octapharma US: Honoraria; Roche: Speakers Bureau; Shire: Speakers Bureau; Sobi: Speakers Bureau; Roche: Speakers Bureau; Shire: Speakers Bureau; Sobi: Speakers Bureau; Octapharma US: Honoraria; Ablynx: Other: Member of Advisory Board, Speakers Bureau; Ablynx: Other: Member of Advisory Board, Speakers Bureau; Kedrion: Consultancy; Grifols: Speakers Bureau; Kedrion: Consultancy; Kedrion: Consultancy; Ablynx: Other: Member of Advisory Board, Speakers Bureau; Grifols: Speakers Bureau; Roche: Speakers Bureau; Roche: Speakers Bureau; Novo Nordisk: Speakers Bureau; Shire: Speakers Bureau; Shire: Speakers Bureau; Sobi: Speakers Bureau; Novo Nordisk: Speakers Bureau; Ablynx: Other: Member of Advisory Board, Speakers Bureau. Coppo:Ablynx: Consultancy. Kremer Hovinga:Ablynx: Other: Member of Advisory Board; Shire: Other: Member of Advisory Board, Research Funding. Metjian:Ablynx: Other: Member of Advisory Board. De La Rubia:Ablynx: Consultancy, Other: Member of Advisory Board. Minkue:Ablynx: Employment. Callewaert:Ablynx: Employment. De Winter:Ablynx: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal